
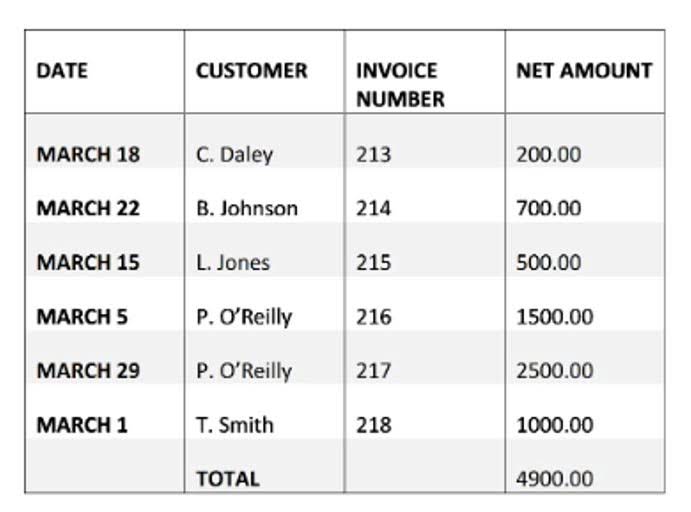
POHR relies on estimated costs and activity levels, so the amount of overhead applied to products during a period will differ from the actual overhead costs incurred. This difference results in either underapplied overhead (actual overhead exceeds applied) or overapplied overhead (applied overhead exceeds actual). These differences are typically adjusted in accounting records at the end of the accounting period to reconcile estimated and actual figures. Historically, Bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.
Advantages of predetermined overhead rate formula
Hence, preliminary, company A could be the winner of the auction even though the labor hour used by company B is less, and units produced more only because its overhead rate is more than that of company A. Use the following data for the calculation of a predetermined overhead rate. Two companies, ABC company, and XYZ company are competing to get a massive order that will make them much recognized in the market. This project is going to be lucrative for both companies but after going over the terms and conditions of the bidding, it is stated that the bid would be based on the overhead rate. This means that since the project would involve more overheads, the company with the lower overhead rate shall be awarded the auction winner.
- According to nineteenth-century science, atoms are the smallest indivisible quantities of matter.
- Lines in the spectrum were due to transitions in which an electron moved from a higher-energy orbit with a larger radius to a lower-energy orbit with smaller radius.
- That is, the company is now aware that a 5-hour job, for instance, will have an estimated overhead cost of $100.
- Treating anelectron as a particle fails to produce a model which can describeall the elements.
- Ernest Rutherford had proposed a model of atoms based on the \(\alpha\)-particle scattering experiments of Hans Geiger and Ernest Marsden.
When is the predetermined manufacturing overhead rate computed?
Niels Bohr is attempting to provide an explanation for the emission spectrum of hydrogen. The photon is released as the electron transitions from an excited state to a lower energy state. The energy change of the electron is equal to the energy of the photon that it releases.
The Enghoff modification of Bohr’s method
- It can help manufacturers know when to review their spending more closely, in order to protect their business’s profit margins.
- Determines the amount of physiological dead space in a person’s lungs to evaluate extent of wasted ventilation.
- Electron total energies are negative, since the electron is bound to the nucleus, analogous to being in a hole without enough kinetic energy to escape.
- Manufacturing overheads are indirect costs which cannot be directly attributed to individual product units and for this reason need to be applied to the cost of a product using a predetermined overhead rate.
- If the job in work in process has recorded actual material costs of 4,640 for the accounting period then the predetermined overhead applied to the job is calculated as follows.
- Figure 5 shows an energy-level diagram, a convenient way to display energy states.
The energy expression for hydrogen-like atoms is a generalization of the hydrogen atom energy, in which Z is the nuclear charge (+1 for hydrogen, +2 for He, +3 for Li, and so on) and k has a value of 2.179 ×× 10–18 J. However, more direct evidence was needed to verify the quantized nature of energy in all matter. In this section, we describe how observation of the interaction of atoms with visible light provided this evidence. By using the predetermined rate product costs and therefore selling prices can be calculated quickly throughout the year without the need to wait for actual overheads to be determined and allocated. In addition while manufacturing overheads might vary seasonally throughout the year, the use of a constant predetermined rate avoids a similar variation in unit product cost. The estimated manufacturing overhead cost applied to the job during the accounting period will be 1,450.
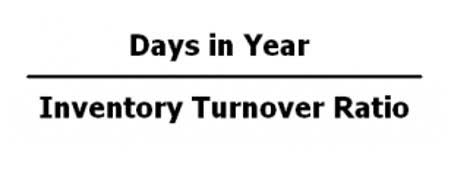
Schwarzschild radius
This is why a predetermined overhead rate is computed to allocate the overhead costs to the production output in order to determine a cost for a product. The predetermined overhead rate is, therefore, usually used for contract bidding, product pricing, and allocation of resources within a company, based on each department’s utilization of resources. The company, having calculated its overhead costs as $20 per labor hour, now has a https://tandemargentina.com/time-value-of-money-tvm-calculator/ baseline cost-per-hour figure that it can use to appropriately charge its customers for labor and earn a profit. That is, the company is now aware that a 5-hour job, for instance, will have an estimated overhead cost of $100. The Rydberg formula for hydrogen gives the exact positions of the spectral lines as they are observed in a laboratory; however, at the beginning of the twentieth century, nobody could explain why it worked so well.
The regional alveolar CO2 varies significantly in the lung because of the regional differences in V/Q ratios; for example even in the healthy lung in a vertical position Brandis gives the apical PACO2 as 28 mmHg and basal PACO2 as 42 mmHg. Additionally, the equation returns all dead space as a result, which means one is unable to determine the specific size of the alveolar and anatomical spaces. Thus far we have explicitly considered only the emission of light by atoms in excited states, which produces an emission spectrum (a spectrum produced by the emission of light by atoms in excited states).
Planetary models
- The electrons do not spiral into the nucleus, as expected classically (accelerated charges radiate, so that the electron orbits classically would decay quickly, and the electrons would sit on the nucleus—matter would collapse).
- After going to its terms and conditions of the bidding, it stated the bid would be based on the overhead rate percentage.
- The actual energy levels cannot be solved analytically for more than one electron (see n-body problem) because the electrons are not only affected by the nucleus but also interact with each other via the Coulomb force.
- In some cases, it had been possible to devise formulas that described the emission spectra.
- When an atom emits light, it decays to a lower energy state; when an atom absorbs light, it is excited to a higher energy state.
- Historically, Bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen.
E is assumed to be negative, because a positive energy is required to unbind the electron from the nucleus and put it at rest at an infinite distance. The PaCO2-ETCO2 difference is a consequence of dead space, as dead space gas dilutes alveolar gas. However this has nothing to do with the measurement of dead space and it was not a part of Fowler’s original experiment.

Bohr’s Solution for Hydrogen

The converse, absorption of light by ground-state atoms to produce an excited state, can also occur, producing an absorption spectrum (a spectrum produced by the absorption of light by ground-state atoms). It is the strongest atomic emission line from the sun and drives the chemistry of the upper atmosphere of all the planets, producing ions by stripping electrons from atoms and molecules. It is completely absorbed by oxygen in the upper stratosphere, dissociating O2 molecules to O atoms which react with other O2 molecules to form stratospheric ozone. The Bohr equation, named after Danish physician pohr equation Christian Bohr (1855–1911), describes the amount of physiological dead space in a person’s lungs. It differs from anatomical dead space as measured by Fowler’s method as it includes alveolar dead space. At the end of the accounting period the applied overhead is compared to the actual overhead and any difference is posted to the cost of goods sold or, if significant, to work in process.
- The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.
- Applied overhead, along with direct materials and direct labor costs, contributes to a good’s total production cost.
- The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.
- Since electrons are integral in chemical reactions, learning more about their behavior–how they move from one shell to another—helps in understanding what that behavior can tell us about their reactivity.
- Businesses monitor relative expenses by having an idea of the amount of base and expense that is being proportionate to each other.
In 1967, the second was defined as the duration of 9,192,631,770 oscillations of the resonant frequency of a cesium atom, called the cesium clock. Research is currently under way to develop the next generation how is sales tax calculated of atomic clocks that promise to be even more accurate. In contemporary applications, electron transitions are used in timekeeping that needs to be exact.
